The data we generate as consultants and scientists may be used to identify potential impacts to human health and the environment and are often used to make costly remediation determinations. TRC’s Quality Assurance system incorporates Quality Control tools such as routine Data Validation (DV) and Data Usability Assessments (DUA) to ensure reliable data are available to inform critical project decisions.
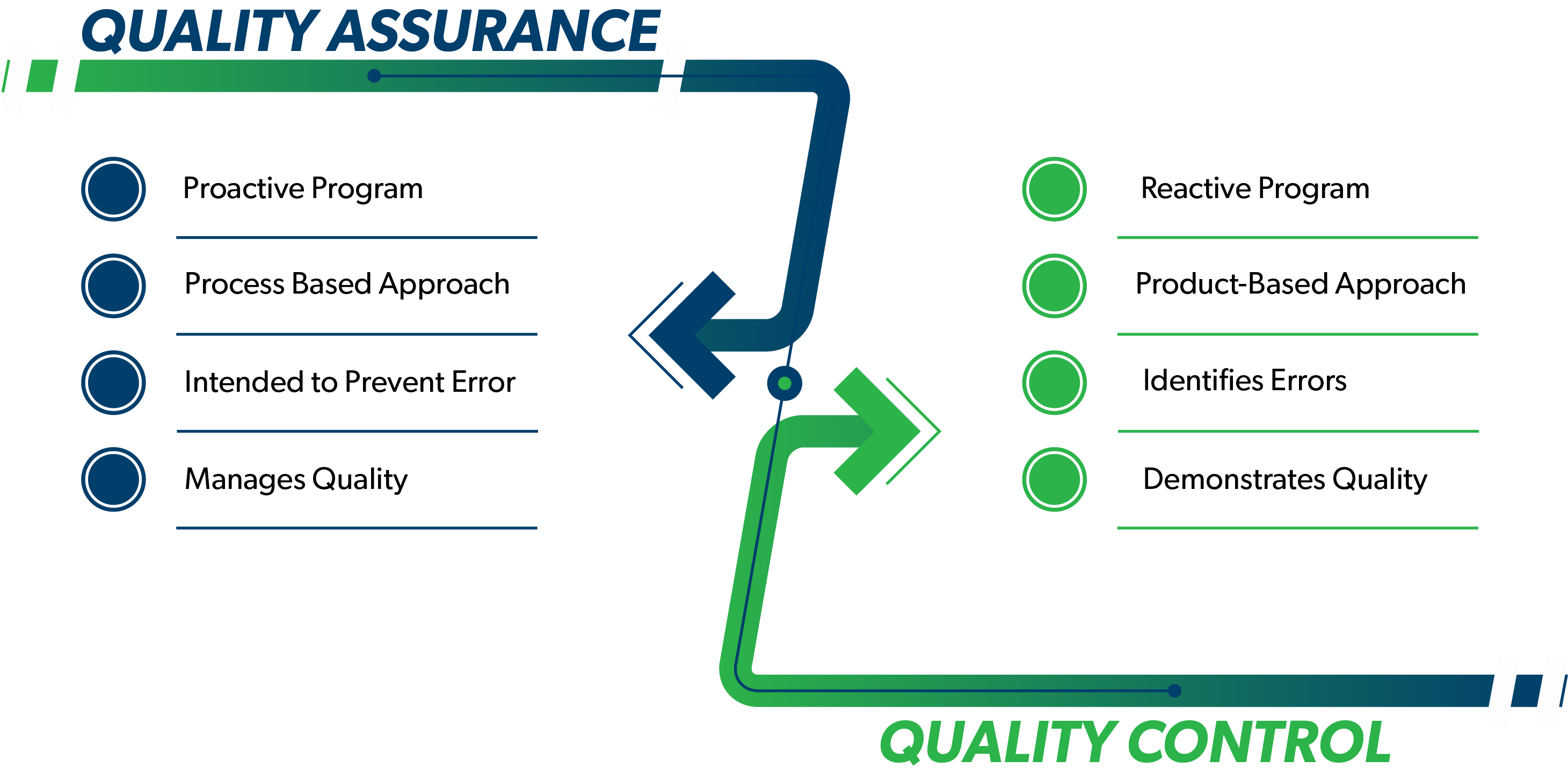
Quality Assurance (QA) and Quality Control (QC) are two elements of a quality management program. Although the terms are frequently used interchangeably, there are important differences distinguishing these terms and practices. QA activities generally involve most of the quality system, while QC is a subset of the QA activities. Understanding the relationship between these two elements allows for a more efficient and effective quality management system. This is especially important when managing data utilized to make critical decisions about a project and how to proceed with the appropriate action to meet client goals.
Related Services

Quality Assurance
QA is part of the quality management system that provides a program to ensure that quality requirements are monitored and maintained. QA is intended to prevent problems by following rules, regulations and standard operating procedures (SOPs). Systematic activities such as QA Management Planning and control charts are implemented within the QA system to provide confidence that a product or service meets all defined requirements for quality.
 Quality Control
Quality Control
QC consists of tools used as part of the quality management system to measure and demonstrate whether the system is maintaining quality requirements. This includes laboratory control samples, laboratory method blanks, field blanks, duplicates, etc. The requirement to perform data validation is in the QA program. Data validation is a QC process used to measure data quality. Based on the results of QC tests, certain actions or modifications to the process(es) may be required. While QA is proactive, QC is considered reactive.
Why Does it Matter?
QC generally identifies concerns after they occur, while QA is intended to prevent problems before they occur. An effective program that leverages both QA and QC practices has many benefits including:
- Increased Customer Confidence – Stakeholders can have confidence that the company they are investing in is effective and trustworthy if it follows robust QA/QC procedures.
- Preventing Mistakes – A robust QA/QC program ensures that the project, product or service is completed correctly.
- Improved Safety – Following both QA/QC management practices prevents accidents and ensures work is performed safely.
- Cost Savings – QA/QC management minimizes data and process errors and rework; products or services are generated more efficiently, and projects are completed more effectively.

TRC Can Help
Project staff should plan early with the laboratory and project chemists/data reviewers for their data deliverable and data review needs. Not sure what level of data review you need or who to ask for DV and DUA needs? TRC’s QA/Chemistry CORE Team can help! We can work with you to understand what is needed for successful achievement of project objectives and help find a qualified staff member to perform the required level of data review.



 Quality Control
Quality Control